Master Data Management in Pharma: Overcome Data Challenges

by Brian Fitzgerald | Last Updated: December 18, 2024 | 1 min read
Data drives every aspect of pharmaceutical innovation. From drug discovery to patient engagement, accurate data fuels critical decisions. Without reliable data, errors in dosage, regulatory delays, and operational inefficiencies become inevitable. In a fast-evolving healthcare landscape, companies that fail to manage their data effectively risk falling behind.
The Role of Master Data Management (MDM) in Pharma
Master Data Management (MDM) is a game-changer for the pharmaceutical industry. It ensures that all company-wide data is accurate, consistent, and accessible in real-time. This holistic approach to Data Management in Pharma eliminates data silos and provides a unified view of operations. By enabling better data visibility, MDM simplifies workflows, accelerates decision-making, and reduces costly errors.
MDM is especially critical for handling regulatory compliance. The pharmaceutical sector must adhere to regulations like FDA 21 CFR Part 11 and GDPR. Regulatory Compliance in Pharma is complex, but MDM provides an audit-ready data framework, reducing compliance risks.
How BirdzAI is Transforming Pharma Data Management
BirdzAI's Master Data Management solution is purpose-built for the unique needs of the pharma industry. By integrating real-time processing, data cleansing, and regulatory compliance features, BirdzAI addresses critical Pharma Data Challenges. Its intuitive platform enables pharmaceutical companies to streamline data, comply with industry standards, and improve operational efficiency. With BirdzAI, organizations are better equipped to drive innovation, ensure compliance, and strengthen their competitive edge.
What is Master Data Management (MDM) in Pharma?
Master Data Management (MDM) is the process of creating a single, unified source of data for an organization. In the pharmaceutical industry, MDM ensures that critical information related to research, clinical trials, supply chains, and patient interactions is accurate, consistent, and accessible across departments. It serves as the foundation for efficient Data Management in Pharma, enabling companies to maintain operational efficiency and regulatory compliance.
Unlike fragmented systems, MDM organizes data from multiple sources into a central repository. This unified approach allows for better decision-making, faster regulatory approvals, and smoother supply chain operations. For pharmaceutical companies, MDM is a strategic asset that reduces errors, eliminates redundancies, and drives data-driven innovation.
How MDM in Pharma Differs from Other Industries
While MDM is essential for all industries, its role in life sciences is far more critical. In sectors like retail or manufacturing, MDM focuses on customer or product data. However, in the pharmaceutical industry, MDM manages highly sensitive information, such as:
- Clinical Trial Data: Ensuring accuracy and traceability of patient information and trial outcomes.
- Regulatory Compliance Data: Supporting adherence to guidelines like FDA’s 21 CFR Part 11 and GDPR.
- Supply Chain Data: Enabling real-time tracking of raw materials and product shipments.

MDM in pharma also faces stricter data governance requirements due to Regulatory Compliance in Pharma. The consequences of non-compliance can be severe, leading to fines, product recalls, or loss of market access. Therefore, MDM in life sciences must be more secure, auditable, and scalable than in other sectors.
Why MDM is Essential for Pharma Companies
Master Data Management (MDM) plays a pivotal role in addressing Pharma Data Challenges. It supports vital operations that directly impact profitability, efficiency, and compliance. Key areas where MDM adds value include:
- Regulatory Compliance: MDM ensures that pharmaceutical companies can maintain accurate audit trails and meet global regulatory standards. It simplifies data validation and reporting, reducing the risk of non-compliance.
- Supply Chain Efficiency: By providing real-time data access, MDM enables better tracking of inventory, raw materials, and product shipments, reducing delays and enhancing transparency.
- Clinical Trials: During trials, accurate and accessible data is crucial. MDM ensures the integrity of patient records, drug efficacy results, and adverse event reports, facilitating faster drug approvals.
Master Data Management is no longer optional for pharmaceutical companies. Its ability to streamline operations, support compliance, and drive innovation makes it a key driver of success in a highly regulated industry. Solutions like BirdzAI offer advanced MDM capabilities tailored for the unique needs of life sciences, making it easier to overcome Pharma Data Challenges and stay ahead of competitors.
Explore More Relevant Articles on P360
- BirdzAI’s Marketing Operations Module Provides Powerful Automations and Digital Build-Out Services
- BirdzAI’s Sales Operations Module Provides Seamless Data Integration, CRM Implementation Assistance and Help Desk Support
- How BirdzAI Makes Field Data Change Requests and Incentive Compensation Easy
- How BirdzAI’s Sales Operations Module Streamlines and Simplifies Alignment and Roster Management
- Revitalize Your Pharmaceutical Commercial Operation with Artificial Intelligence (AI)!
Challenges in Data Management for Pharma Companies
Overview of Data Management Challenges in the Pharma Industry
Data is the driving force behind pharmaceutical innovation. From drug development to patient care, accurate data is essential for every process. However, managing vast amounts of data across multiple departments presents significant hurdles. The presence of siloed systems, data quality issues, regulatory demands, and security risks creates a complex landscape.
Without an effective Master Data Management (MDM) system, these challenges slow down operations, increase compliance risks, and reduce overall efficiency. Below are the key Pharma Data Challenges that affect life sciences companies and how MDM can help address them.
1. Data Silos
Data silos are isolated data sets that are not accessible across departments. In pharma, research and development (R&D), marketing, regulatory affairs, and supply chain teams often work with separate data systems. This lack of connection leads to:
- Delayed Decision-Making: Teams cannot access real-time data from other departments.
- Redundant Data: Duplicate records lead to inconsistencies and misaligned reports.
- Compliance Risks: Regulatory audits become more challenging due to disjointed data.
How MDM Resolves Data Silos
MDM unifies data from multiple departments into a central, shared repository. This system ensures seamless communication and promotes collaboration between R&D, regulatory affairs, and marketing teams. With cross-department access, pharma companies can reduce compliance risks and improve operational efficiency. BirdzAI’s MDM solution eliminates silos, enabling real-time data sharing for better decision-making.
2. Data Quality Issues
Inaccurate data is one of the most pressing Pharma Data Challenges. Flawed or incomplete data can affect every aspect of the pharma value chain. Clinical trials, patient engagement, and regulatory filings all rely on clean, validated data. The effects of poor data quality include:
- Clinical Trial Delays: Patient records and trial outcomes must be accurate for regulatory approvals.
- Patient Safety Risks: Inaccurate dosage data could harm patients or delay drug launches.
- Regulatory Setbacks: Inconsistent data increases the likelihood of non-compliance penalties.
How MDM Improves Data Quality
With Master Data Management, data validation, cleansing, and enrichment become standard processes. MDM identifies and removes duplicate, outdated, or incorrect data. This ensures that clinical trial outcomes are reliable and patient safety is prioritized. BirdzAI’s data cleansing tools reduce human errors and maintain data accuracy for better operational outcomes.
3. Regulatory Compliance Requirements
Regulatory compliance in pharma is one of the most stringent in any industry. Companies must meet guidelines like:
- FDA’s 21 CFR Part 11: Requires secure, traceable electronic records and signatures.
- GDPR: Imposes strict privacy regulations for managing patient data.
- ISO Standards: Set quality benchmarks for data integrity and risk management.
How MDM Supports Regulatory Compliance
MDM enables companies to maintain an audit-ready environment. Automated data tracking and validation are built into the system, making it easier to meet regulatory guidelines. Compliance teams have access to a unified view of data, which supports faster responses to audits. BirdzAI’s MDM solution ensures that regulatory submissions are complete, accurate, and on time.
4. Integration of Diverse Data Sources
Pharma companies face data fragmentation after mergers, acquisitions, and partnerships. Different systems collect data in different formats, creating challenges like:
- Data Incompatibility: Systems use unique formats, making data exchange difficult.
- Data Duplication: M&A activities introduce duplicate records, leading to inconsistencies.
- Delayed Reporting: Disparate data slows down reports for regulatory filings and strategic planning.
How MDM Harmonizes Diverse Data Sources
MDM consolidates and standardizes data from different sources into a single, cohesive format. BirdzAI’s Master Data Management system automatically identifies and resolves discrepancies, enabling real-time reporting. As new data sources emerge from acquisitions or partnerships, BirdzAI seamlessly integrates them into the master system.
5. Data Security & Privacy
Data security is a growing concern as more patient records, clinical trial data, and proprietary research are digitized. Security lapses can expose sensitive data, leading to:
- Data Breaches: Cyberattacks can compromise clinical trial data or patient information.
- IP Theft: Theft of drug formulas and research results can impact profitability.
- Compliance Fines: Breaches of GDPR or FDA regulations result in heavy financial penalties.
How BirdzAI Ensures Secure MDM
BirdzAI’s Master Data Management system prioritizes data security and privacy. With multi-layer encryption, user authentication, and access controls, BirdzAI protects sensitive pharmaceutical data from unauthorized access. The platform also supports GDPR and FDA compliance, ensuring that patient data is handled securely and transparently.
BirdzAI’s Approach to Master Data Management (MDM) in Pharma
Pharma companies face complex Pharma Data Challenges due to fragmented systems, regulatory compliance, and the need for accurate data. BirdzAI’s Master Data Management(MDM) solution addresses these issues with precision. Purpose-built for the life sciences industry, BirdzAI provides a unified, secure, and efficient platform for Data Management in Pharma.
With BirdzAI, pharmaceutical companies can streamline data, improve operational efficiency, and ensure Regulatory Compliance in Pharma. Key features like real-time data processing, data cleansing, advanced analytics, and secure integration make BirdzAI a preferred MDM solution.
Key Features of BirdzAI’s MDM Solution
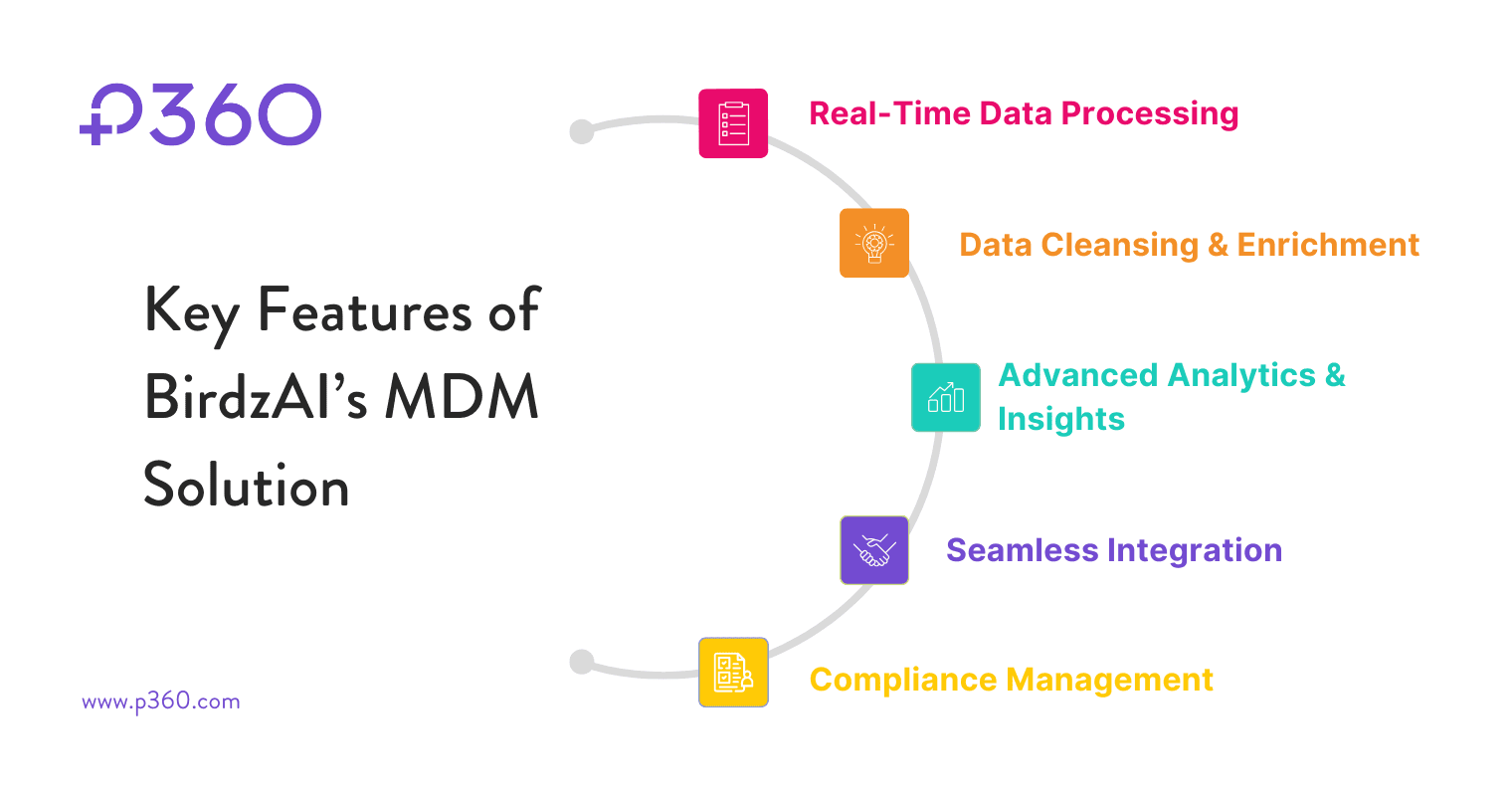
1. Real-Time Data Processing
Access to real-time data is critical for fast, informed decision-making in the pharmaceutical industry. BirdzAI’s Master Data Management processes data instantly, ensuring that information is always up-to-date. This feature plays a vital role in:
- Clinical Trials: Real-time access to trial data helps researchers track patient outcomes, accelerating approvals.
- Supply Chain Decisions: Live updates on inventory and shipping enable quicker responses to potential disruptions.
With BirdzAI’s MDM, pharma companies can reduce delays, avoid errors, and ensure product availability in the market. This real-time approach supports faster drug approvals and enhanced market responsiveness.
2. Data Cleansing & Enrichment
Data inconsistencies and duplicates are common Pharma Data Challenges. Poor data quality affects clinical trials, patient safety, and compliance efforts. BirdzAI’s MDM automates data cleansing, validation, and enrichment processes. Key benefits include:
- Elimination of Duplicate Data: Removes redundancy across R&D, marketing, and regulatory departments.
- Data Consistency: Standardized data formats ensure smooth sharing between departments and faster audits.
Accurate and enriched data enables smarter decision-making. BirdzAI's cleansing tools ensure that every dataset is ready for analysis, improving efficiency in clinical research and supply chain management.
3. Advanced Analytics & Insights
BirdzAI enhances Data Management in Pharma by transforming raw data into meaningful insights. Powered by Artificial Intelligence (AI) and Machine Learning (ML), BirdzAI’s MDM generates actionable insights for better decision-making. This approach enables:
- Supply Chain Optimization: AI-driven forecasts help predict demand, prevent stockouts, and improve production schedules.
- R&D Insights: ML algorithms identify patterns in research data, helping pharma companies develop more effective drug formulations.
These capabilities provide a significant competitive edge. By leveraging BirdzAI's predictive analytics, pharmaceutical firms can reduce costs, accelerate innovation, and create a data-driven culture.
4. Seamless Integration with Existing Systems
Pharma companies often use legacy systems that are difficult to replace. Integrating these systems with new MDM platforms is a significant hurdle. BirdzAI's MDM Solution for Pharma offers seamless compatibility with existing software, ensuring no disruption to business operations.
Key advantages of this integration approach include:
- Reduced Downtime: Systems continue to function during the migration process.
- Minimal Training Required: BirdzAI’s user-friendly interface allows non-technical users to access MDM features easily.
With BirdzAI, pharma companies can modernize their data management process without risking operational delays. Legacy systems can be connected, enabling all departments to access accurate, centralized data.
5. Compliance Management
Pharmaceutical companies face strict regulatory scrutiny under frameworks like:
- FDA’s 21 CFR Part 11 Requires traceability of electronic records and secure digital signatures.
- GDPR: Imposes stringent rules on personal data protection, including patient records and clinical trial data.
BirdzAI addresses these compliance challenges with built-in workflows for Regulatory Compliance in Pharma. Automated audit trails ensure that data is always ready for inspections. This feature simplifies regulatory audits, as BirdzAI automatically tracks and documents every change in the system.
With BirdzAI, companies can ensure compliance without manual effort. Compliance workflows and automated audit trails allow regulatory teams to focus on more strategic tasks, reducing risk and saving time.
6. Security & Privacy Protocols
Pharmaceutical data is among the most sensitive in the world. Research data, clinical trial information, and patient health records are prime targets for cyberattacks. BirdzAI ensures complete data security by adopting a multi-layered security approach.
How BirdzAI Protects Pharma Data:
- Data Encryption: All data is encrypted in transit and at rest, ensuring that sensitive data is inaccessible to unauthorized users.
- Access Controls: Role-based access restricts data access based on user roles, protecting sensitive information.
- Audit Logs: Detailed logs track who accessed the data and when, ensuring traceability and accountability.
By addressing the growing demand for data privacy, BirdzAI strengthens its position as a secure MDM Solution for Pharma. Companies can safeguard patient data, research, and intellectual property from internal and external threats.
Business Impact of MDM on Pharma Companies
1. Improved Data Accuracy
Accurate data is essential for the success of clinical trials, regulatory submissions, and supply chain operations. Errors in patient records, dosage details, or shipment tracking can result in costly delays and safety risks. Master Data Management (MDM) eliminates these issues by ensuring a single, consistent source of truth for all data.
With BirdzAI’s MDM, manual errors are reduced, and data quality is maintained across departments. Clinical trial data becomes more reliable, making it easier for pharmaceutical companies to secure regulatory approvals. Supply chain tracking is also improved, as all stakeholders have access to accurate, real-time information on inventory, shipping, and distribution.
2. Informed Decision-Making
Decisions in the pharmaceutical industry are driven by data. However, when data is fragmented, leaders face delays and confusion. Master Data Management creates a unified view of company-wide data, allowing decision-makers to act quickly and with confidence.
BirdzAI’s MDM provides a "single source of truth," enabling pharmaceutical leaders to access real-time, accurate data from R&D, regulatory affairs, and supply chain teams. By using this consolidated data, companies can predict market trends, optimize production, and make faster decisions during clinical trials.
3. Streamlined Operations
Manual data tasks such as data entry, validation, and cleansing consume significant time and resources. BirdzAI’s MDM solution for pharma automates these processes, allowing teams to focus on higher-value tasks.
Automation eliminates the need for repetitive, labor-intensive processes, reducing human error. With streamlined operations, pharmaceutical companies can improve productivity, optimize resource allocation, and achieve better data consistency. This operational efficiency ultimately leads to faster drug development and enhanced patient outcomes.
4. Faster Time-to-Market
Speed is critical in the pharmaceutical industry, especially during drug development and clinical trials. Delays in regulatory approvals or supply chain disruptions can cause significant financial loss. Master Data Management accelerates time-to-market by enabling faster, more efficient processes.
With BirdzAI’s MDM, regulatory documentation is prepared accurately, reducing back-and-forth communication with regulatory bodies. Supply chain data is also streamlined, ensuring raw materials and finished products reach their destinations on time. By reducing delays in regulatory submissions and distribution, BirdzAI helps pharmaceutical companies achieve faster market entry for new drugs.
5. Regulatory Compliance and Peace of Mind
Pharmaceutical companies must adhere to strict regulatory requirements, including FDA’s 21 CFR Part 11 and GDPR. Compliance failures can result in costly penalties and reputational damage. BirdzAI’s MDM offers compliance-ready workflows that simplify adherence to regulatory standards.
With BirdzAI, every data change is automatically tracked, creating an audit-ready system. Compliance audits become faster and less burdensome because all data changes are documented. Regulatory risks are significantly reduced, giving pharma companies peace of mind. This compliance-driven approach ensures smooth operations during inspections and audits, while also maintaining patient data privacy and security.

Why BirdzAI is the Ideal MDM Solution for Pharma
The pharmaceutical industry faces unique challenges in managing data. Regulatory compliance, data security, and operational efficiency are key concerns. BirdzAI addresses these needs with a purpose-built Master Data Management (MDM) solution designed exclusively for the life sciences sector. Its advanced features make it the go-to solution for overcoming Pharma Data Challenges.
1. Industry-Specific Focus
Unlike generic MDM platforms, BirdzAI is tailored for Data Management in Pharma. Its design addresses specific industry needs, such as clinical trial data management, patient data security, and regulatory compliance. This focus ensures that critical pharma workflows are fully supported. From R&D to regulatory submissions, BirdzAI adapts to every stage of the product lifecycle, helping companies streamline processes and maintain compliance.
2. Scalable Architecture
Pharmaceutical companies operate on a global scale, and their data management systems must do the same. BirdzAI’s scalable architecture allows for seamless expansion as the business grows. Whether supporting a single R&D facility or managing supply chain data across multiple locations, BirdzAI scales to meet the need. This flexibility ensures continuous support as new data sources and regulatory requirements are introduced.
3. Intuitive User Interface
Data management tools can be complex, but BirdzAI prioritizes user experience. Its intuitive interface is designed for both technical and non-technical users. Minimal training is required, allowing employees in R&D, regulatory, and supply chain teams to access, manage, and visualize data with ease. The platform’s simplicity reduces reliance on IT teams, saving time and boosting productivity.
4. Multi-Layered Security
Data privacy and protection are essential for pharmaceutical companies. BirdzAI’s Master Data Management system includes multi-layered security features to safeguard clinical, patient, and proprietary data. Key security measures include:
- Data Encryption: Ensures that sensitive data remains secure both in transit and at rest.
- Role-Based Access: Limits access to critical data based on user roles, protecting sensitive information.
- Audit Logs: Tracks every data change, ensuring full traceability and support for Regulatory Compliance in Pharma.
With these security protocols, BirdzAI enables companies to meet the requirements of regulations like FDA’s 21 CFR Part 11 and GDPR. By protecting sensitive data, BirdzAI ensures compliance and builds trust with stakeholders.
Future of MDM in Pharma
The future of Master Data Management (MDM) in the pharmaceutical industry is being shaped by emerging technologies. Innovations like Artificial Intelligence (AI), Machine Learning (ML), and Blockchain are transforming how Data Management in Pharma is approached. These technologies offer new possibilities for improving accuracy, efficiency, and regulatory compliance.
1. Role of Emerging Technologies in MDM
Artificial Intelligence (AI) and Machine Learning (ML)
AI and ML are revolutionizing Master Data Management by enabling automated data analysis, anomaly detection, and predictive insights. These technologies allow MDM platforms like BirdzAI to:
- Detect Data Anomalies: Identify duplicate or incorrect data automatically.
- Predict Market Trends: Use historical data to predict supply chain fluctuations.
- Improve Clinical Trials: Identify patient recruitment patterns and optimize study outcomes.
By leveraging AI and ML, BirdzAI helps pharmaceutical companies enhance data quality and gain valuable insights. These technologies ensure faster, data-driven decisions in R&D, regulatory submissions, and supply chain management.
Blockchain for Data Security
Blockchain is reshaping Data Management in Pharma by adding a layer of transparency and security. It ensures that each data entry is immutable and traceable, which is crucial for regulatory compliance. By using blockchain, BirdzAI offers:
- Tamper-Proof Audit Trails: Every change in data is recorded, providing an indisputable audit trail for compliance.
- Data Privacy: Patient information is stored securely, supporting Regulatory Compliance in Pharma.
- Supply Chain Transparency: Ensures that raw material tracking and product delivery records are fully transparent.
Blockchain's role in MDM will continue to grow, especially in response to the increasing focus on data privacy and compliance.
2. BirdzAI’s Roadmap for Next-Gen MDM Features
The future of BirdzAI is focused on next-generation MDM features that will redefine Data Management in Pharma. Planned upgrades include:
- Predictive Analytics: Advanced AI-driven insights for forecasting demand and optimizing supply chains.
- Collaborative Data Spaces: Secure environments for research collaborations where data can be shared without breaching compliance.
- Smart Automation: Tools for automating regulatory submissions, ensuring faster compliance with FDA and GDPR guidelines.
- Real-Time Data Visualization: Intuitive dashboards that provide real-time updates on clinical trials, patient data, and operational KPIs.
BirdzAI's roadmap reflects the industry's push for smarter, faster, and more secure MDM solutions. These innovations will help pharma companies stay ahead of Pharma Data Challenges while ensuring Regulatory Compliance in Pharma.
Conclusion
The role of Master Data Management (MDM) in the pharmaceutical industry is more critical than ever. With the growing need for data-driven decision-making, regulatory compliance, and operational efficiency, MDM has become a strategic priority. By addressing key Pharma Data Challenges, MDM ensures data accuracy, simplifies compliance, and accelerates time-to-market for new drugs.
Effective Data Management in Pharma helps companies overcome issues related to data silos, regulatory compliance, and supply chain inefficiencies. BirdzAI stands out as a purpose-built solution that caters to the unique needs of the pharmaceutical industry. Its advanced features, such as real-time data processing, data cleansing, and AI-driven insights, enable companies to operate more efficiently and meet regulatory standards.
With BirdzAI, pharma companies can reduce compliance risks, improve data security, and gain a competitive edge. The platform's seamless integration, automated compliance workflows, and multi-layered security make it a comprehensive MDM solution for pharma.
If you are ready to streamline Data Management in Pharma, ensure compliance, and drive innovation, BirdzAI is the solution you need. Discover how BirdzAI can simplify your MDM journey and overcome even the most complex Pharma Data Challenges. Take the next step toward secure, efficient, and future-proof data management for your pharmaceutical operations. Contact BirdzAI today and experience the future of MDM in pharma.