Comprehensive Guide for Pharmaceutical Startups: Commercialization Steps

by Jerlyn Rodrigues | Last Updated: July 11, 2024 | 1 min read
Making the complex journey from groundbreaking pharmaceutical discovery to market introduction is no easy task. For startups in particular, the journey can be fraught with challenges, ranging from working with limited capital to coping with stringent regulatory requirements and grappling with intense market conditions. There’s a lot that can happen between phase zero to launch, and that’s why having a solid commercialization plan is so important. It’s the playbook that shows investors and other stakeholders how your novel discovery can become a viable product that will make a real impact on patient lives.
But what is commercialization?
Simply stated, commercialization is the process through which a new drug, biological product or device moves from the bench to the bedside. It marks the transition from rigorous scientific research to strategic business operations. For businesses in the pharmaceutical industry, successful commercialization is not just about achieving FDA approval; it’s about ensuring the new product achieves commercial viability. This involves navigating multiple stages, each playing a crucial role in determining the product’s ultimate success.
Here are the main steps involved:
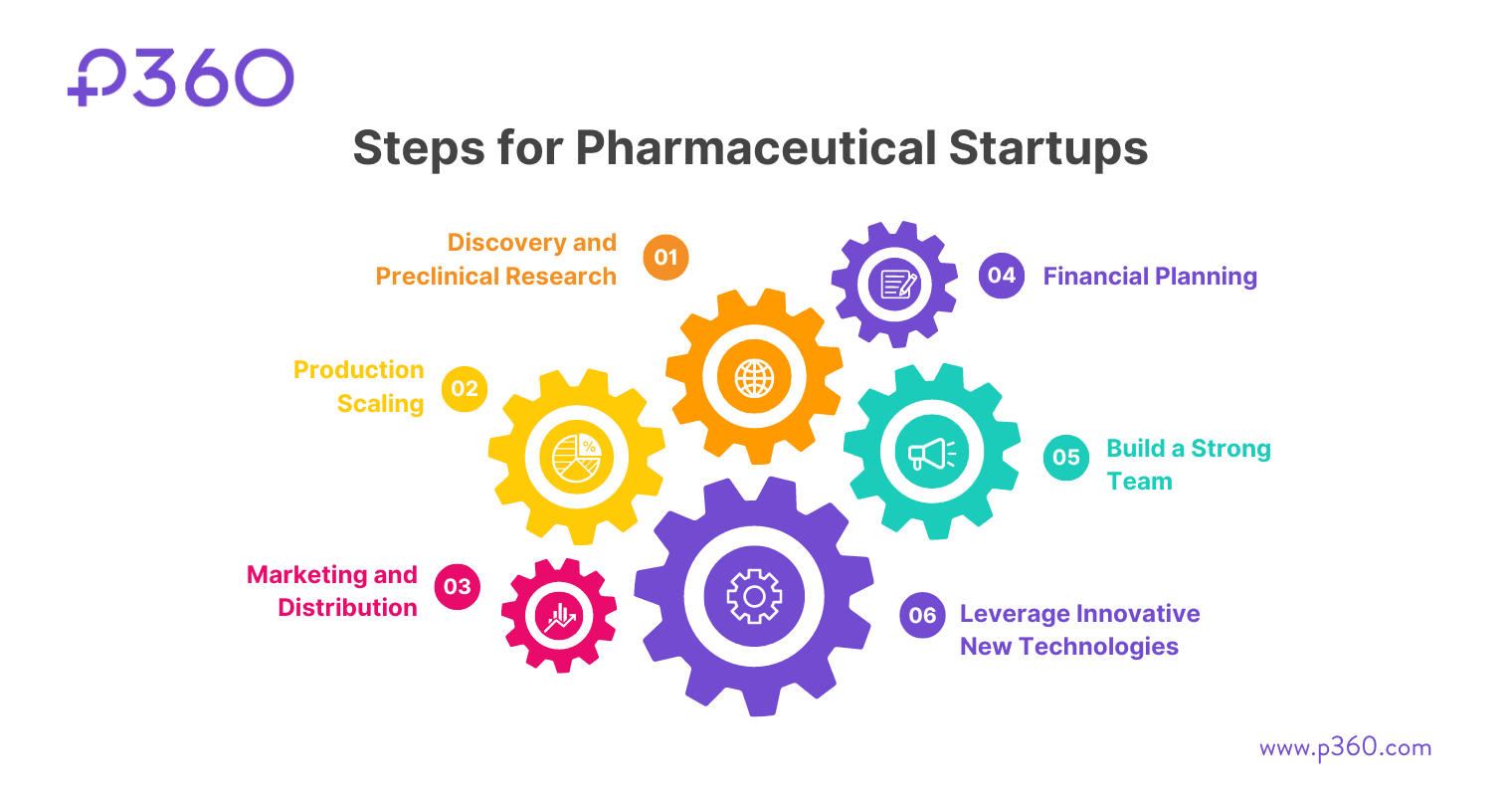
Step 1: Discovery and Preclinical Research
For pharmaceutical companies, the path to commercialization begins in the laboratory with the discovery of a novel therapeutic or diagnostic compound. Preclinical research involves extensive laboratory and animal studies to assess the compound’s efficacy and safety. This stage is critical for establishing a solid scientific foundation for the drug.
The first and perhaps most daunting hurdle at this stage is gaining regulatory approval. Regulatory bodies like the FDA (U.S. Food and Drug Administration) or EMA (European Medicines Agency) have stringent requirements that your product must meet before it can be marketed. For pharmaceutical startups in the U.S., having someone on the team who understands the FDA approval process is critical. Following is a quick overview.
1. Preclinical Research: The first step in the FDA approval process is preclinical research, which involves laboratory and animal testing to evaluate the drug’s safety and biological activity. This includes in vitro (test tube or cell culture) and in vivo (animal) studies to gather preliminary efficacy, toxicity and pharmacokinetic data. Formulation development is also included here, whereby scientists develop a drug formulation that ensures consistency and stability.
Preclinical research aims to generate sufficient data to support a drug’s potential safety and efficacy, setting the stage for human testing.
2. Investigational New Drug (IND) Application: Before initiating clinical trials in humans, a pharmaceutical company must submit an Investigational New Drug (IND) application to the FDA. The IND application includes information on the drug's composition, manufacturing process and plans for human clinical trials. The purpose of this is that it allows the FDA to review the proposed clinical trial design and ensures that the rights and safety of trial participants are protected.
-
Clinical Trials: Clinical trials are conducted in three phases to evaluate the drug's safety, efficacy, and optimal dosage in humans. They include:
-
Phase I: The drug will be tested in a small group (20-80) of healthy volunteers or patients to assess safety, dosage range and side effects.
-
Phase II: Testing is expanded to a larger group (100-300) of patients to evaluate efficacy and further assess safety.
-
Phase III: Large-scale trials begin (1,000-3,000+ patients) to confirm efficacy, monitor side effects and compare the drug to standard treatments.
The objective of clinical trials is to gather robust data on the drug's safety and efficacy, providing the evidence needed for FDA approval.
3. New Drug Application (NDA) Submission: Upon successful completion of clinical trials, the pharmaceutical company submits a New Drug Application (NDA) to the FDA for review. The NDA must Include all preclinical and clinical trial data, along with detailed information on the drug’s pharmacology, toxicology, manufacturing process and labeling. It must also address the FDA’s requirements for safety, efficacy and manufacturing quality.
The NDA submission formally requests FDA approval to market the new drug, presenting all necessary evidence to demonstrate its benefits outweigh any risks.
-
FDA Review and Approval: The FDA conducts a thorough review of the NDA, which involves evaluating the submitted data, inspecting manufacturing facilities and considering the drug's risk-benefit profile. Key Actions include:
-
Advisory Committee Review: The FDA may convene an advisory committee of independent experts to review the data and provide recommendations.
-
Labeling Discussions: Work with the FDA to finalize the drug's labeling, including prescribing information and safety warnings.
-
Facility Inspections: Undergo inspections of manufacturing facilities to ensure compliance with Good Manufacturing Practices (GMP).
The objective of the FDA review is to confirm that the drug is safe and effective for its intended use and that it meets all regulatory standards.
4. Post-Marketing Surveillance: After FDA approval and market launch, ongoing monitoring of the drug's safety and effectiveness is crucial. This includes Phase IV Trials, which are post-marketing studies that gather additional data on long-term safety and efficacy. To do this well, it’s important to implement a robust system for monitoring and reporting adverse events and side effects. Startups should work with the FDA to address any emerging safety concerns and update labeling as needed.
The goal of post-marketing surveillance is to ensure the drug’s continued safety and efficacy in the general population and promptly address any new risks.
Explore More Relevant Articles on P360
- The BirdzAI Marketing Operations Module Features Seamless Data Integration, Deep Analytics and Dynamic Reporting
- BirdzAI’s Marketing Operations Module Provides Powerful Automations and Digital Build-Out Services
- BirdzAI’s Sales Operations Module Provides Seamless Data Integration, CRM Implementation Assistance and Help Desk Support
- How BirdzAI Makes Field Data Change Requests and Incentive Compensation Easy
- How BirdzAI’s Sales Operations Module Streamlines and Simplifies Alignment and Roster Management
Step 2: Production Scaling
Once regulatory approval is secured, the next step is to scale up production. This involves transitioning from small-scale laboratory production to large-scale manufacturing. Startups without manufacturing capabilities should consider partnering with an established contract development and manufacturing organization (CDMO) to leverage their expertise and infrastructure. Be sure that your chosen partner has implemented robust quality control measures to ensure consistency and compliance with regulatory standards.
Startups should also build redundancies by forging relationships with multiple vendors. It’s important to have a reliable supply chain, especially when the timely availability of raw materials and components is critical.
Step 3: Marketing and Distribution
With production underway, the focus shifts to marketing and distribution. The goal is to create awareness and demand for your product while ensuring it reaches the intended customers efficiently. Your first step should be conducting thorough market research, which will help identify target demographics, market opportunities and competitive threats. This will also help inform the development of a comprehensive marketing strategy that includes digital marketing, traditional advertising and public relations.
During this phase, startups should also look to establish distribution channels that align with their product’s target market. Effective partnerships with pharmacies, hospitals, purchasing groups or direct-to-consumer models can help startups scale quickly and efficiently.
Step 4: Financial Planning
Effective financial planning is crucial for the successful commercialization of pharmaceutical products. Startups often face significant financial challenges, including securing funding and managing cash flow. As the saying goes, companies should avoid putting “all their eggs in one basket.” Founders should explore various funding options, including venture capital, grants, and strategic partnerships.
Developing a detailed budget that outlines projected costs and revenue streams will help inform how much runway a company will need to make it to a commercial launch. This should be supported by a robust financial management system to monitor expenses and ensure sustainable growth.
Step 5: Build a Strong Team
The commercialization process requires a diverse set of skills and expertise. Building a strong, multidisciplinary team can significantly enhance your chances of success. Startups should start hiring for key roles as soon as possible, including regulatory experts, manufacturing specialists, marketing professionals and financial analysts. They should also invest in ongoing training and development programs to keep teams updated on industry trends and best practices.
Executives should also work to foster a collaborative and innovative work culture that encourages problem-solving and continuous improvement. This is a critical step for those hiring during a competitive job market, where employee retention is key.
Step 6: Leverage Innovative New Technologies
Leveraging the right technologies can streamline the commercialization processes, reduce costs and accelerate time to market. For example, AI and ML are revolutionizing drug discovery and development. These technologies can analyze vast datasets to identify potential drug candidates, predict their efficacy, and optimize clinical trial designs.
Startups can use AI-powered platforms to screen large libraries of compounds quickly, predict drug-target interactions and identify biomarkers for personalized medicine. AI and ML can also enhance clinical trials by automating patient recruitment and retention, monitoring patient adherence and detecting adverse events in real-time and analyzing trial data to identify trends and insights rapidly.
Digital health technologies, such as Swittons IoT-enabled smart devices and the ZING Engagement Suite can also play a crucial role in the commercialization of pharmaceutical products. These technologies enable startups to monitor patients remotely during clinical trials, ensuring adherence and capturing real-time health data. They can also boost adherence by delivering personalized health information and reminders, providing virtual support and education and tracking medication adherence and providing feedback to patients and healthcare providers.
Additionally, advanced data and analytics platforms such as BirdzAI can help pharmaceutical startups optimize their commercial strategies. By analyzing large datasets from various sources, startups can identify trends and patterns in disease prevalence and treatment outcomes, optimize pricing and market access strategies and predict market demand and tailor marketing efforts accordingly. Data and analytics can also enhance decision-making by providing actionable insights into patient populations and treatment pathways, identifying potential risks and opportunities and supporting evidence-based decision-making across the product lifecycle.
Case Study: Success Stories
To illustrate the importance of these steps, let’s examine some successful pharmaceutical startups that successfully navigated the commercialization process.
Case Study 1
- Moderna: Moderna's meteoric rise to prominence can largely be attributed to its pioneering work in messenger RNA (mRNA) technology. Initially, mRNA technology was a relatively unexplored area within biotechnology. However, Moderna saw its potential for creating vaccines that could be developed and produced at unprecedented speeds.
One key factor behind Moderna's success was its ability to form strategic partnerships. Early in the pandemic, Moderna partnered with the National Institute of Allergy and Infectious Diseases (NIAID) in the United States to expedite the development of its vaccine. This collaboration allowed them to quickly move from preclinical trials to human trials. Additionally, Moderna secured significant funding from various governmental and private entities. This financial backing was crucial for scaling up manufacturing capabilities and ensuring the vaccine could be distributed globally.

Case Study 2
- BioNTech: BioNTech's partnership with Pfizer is a sterling example of how collaboration can accelerate innovation. By pooling resources, expertise, and infrastructure, the two companies were able to bring their mRNA COVID-19 vaccine to market in record time. Pfizer's established global manufacturing and distribution network complemented BioNTech's innovative research, making the partnership a perfect synergy.
BioNTech's success also underscores the importance of having robust research and manufacturing capabilities. While BioNTech focused on the vaccine’s research and development, Pfizer took charge of the large-scale manufacturing and distribution aspects. This division of labor allowed each company to play to its strengths, optimizing the overall process.
Effective communication with regulatory bodies was another cornerstone of BioNTech's success. The company worked closely with the European Medicines Agency (EMA) and the FDA, providing all necessary data and documentation to expedite the approval process. This proactive approach helped them navigate the complex regulatory landscape efficiently.
When Should Pharmaceutical Startups Start Planning for Commercialization?
For pharmaceutical startups, the timing of their commercialization efforts can make or break their success. And one of the cardinal rules is to start planning for commercialization as early as possible. Ideally, this should begin during the preclinical phase of drug development. While this might seem premature to some, the complexities involved in bringing a pharmaceutical product to market necessitate early preparation.
Here are some reasons why starting early is a good idea:
-
Regulatory Hurdles: Navigating the labyrinthine regulatory landscape is perhaps the most daunting aspect of pharmaceutical commercialization. The Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in Europe, and other regulatory bodies worldwide have stringent requirements that must be met before a product can be marketed. Understanding these requirements early on can help startups design their clinical trials and other studies to generate the necessary data for regulatory approval.
-
Market Research: Understanding the market landscape is crucial for any business, and pharmaceutical startups are no exception. Early market analysis helps identify potential competitors, target demographics, pricing strategies, and market entry barriers. This information is invaluable for developing a robust commercialization strategy.
-
Funding and Investment: Commercialization is an expensive endeavor. From conducting clinical trials to scaling up manufacturing, the costs can be staggering. Early planning allows startups to map out their funding needs and approach investors with a clear, well-thought-out plan. This increases the likelihood of securing the necessary financial backing.
Potential Pitfalls to Avoid
While early and thorough planning is essential, there are several common pitfalls that pharmaceutical startups should be aware of:
-
Overlooking Regulatory Requirements: Failing to understand and comply with regulatory requirements can lead to costly delays and even derail the entire commercialization effort. Startups must stay abreast of regulatory changes and ensure that their development processes are in line with current guidelines.
-
Underestimating Costs: Commercialization is a capital-intensive process. Startups often underestimate the costs involved, leading to funding shortfalls. A detailed budget that accounts for all potential expenses is crucial.
-
Neglecting Market Research: Startups risk developing products that do not meet the needs of their target audience without a thorough understanding of the market. Comprehensive market research is essential for aligning product development with market demand.
-
Inadequate Risk Management: The pharmaceutical industry is inherently risky, with numerous factors that can impact a startup's success. Implementing a robust risk management strategy can help identify potential threats and develop contingency plans.
Final Thoughts
Commercialization is a multifaceted process that goes beyond scientific discovery. It requires meticulous planning, strategic execution, and collaboration across various functions. For pharmaceutical startups, understanding the complexities involved and preparing accordingly can significantly enhance the chances of bringing a new drug to market successfully. However, by focusing on each stage of commercialization and leveraging available resources, startups can transform their groundbreaking discoveries into impactful, market-ready products.
Are you ready to take your pharmaceutical discovery to the next level? Connect with us today to see how Swittons, ZING and BirdzAI can significantly enhance your ability to bring your products to market successfully.